The neutral atom chlorine Z17 for instance has 17 electrons. Chlorine is found in the group 17 the halogens on the periodic table.

Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry
Yes in fact its a pretty easy pattern once youve learned a few rules.

. LIGHT ELECTRON CONFIGURATION AND PERIODIC TRENDS 3. 26 full pdfs related to this paper. Electron Configuration Pattern - 17 images - iron orbital and bonding info chemistry notes on atomic structure electron electron waves.
The first 2 elements fill the 1s level. Using the periodic table and assuminga regular filling pattern give the full and condensed electron configurations partial orbital diagrams showing valence electrons only and number of inner electrons for the following elements. V 1s2 6 2s2 2p 3s2 3p6 4s23d3 4.
The two columns on the leftthe alkali metals and alkaline earthsshow the addition of 1 and 2 electrons into s type subshells. The electron configurations are written in the noble gas notation. Chlorine has an electron configuration of 1s2 2s2 2p6 3s2 3p5.
Unreactive due to electron configuration ns2np6 except He 1s2 Main group elements tend to gain or lose electrons to become isoelectronic same valence electron configuration as. What is the electronic configuration of chlorine. The electron pattern for neon for instance is 1s 2 2s 2 2p 6.
Electron Configuration Li 1 3 1 1s2 2s1 N 5A 7 5 1s2 2s2 2p3 F 7A 9 7 1s2 2s2 2p5 Ne 8A 10 8 1s2 2s2 2p6 Na 1 11 1 1s2 2s2 2p6 3s1 Mg 2 12 2 1s2 2s2 2p6 3s2 Al 3A 13 3 1s2 2s2 2p6 3s2 3p1 Cl 7A 17 7 1s2 2s2 2p6 3s2 3p5 Ar 8A 18 8 1s2 2s2 2p6 3s2 3p6 K 1 19 1 1s2 2s2 2p6 3s2 3p6 4s1 Ca 2 20 2 1s2 2s2 2p6 3s2 3p6 4s2 Br 7A 35 7 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. One of the many patterns contained in the periodic table is that of electron configuration. The Periodic Table The pattern of elements in the periodic table reflects the progressive filling of electronic orbitals.
The names of groups and periods on the periodic chart are alkali metals alkaline earth metals transition metals halogens and noble gases. Ge 1s 22s 2p63s 3p64s23d104p2 PART B SHORTHAND ELECTRON CONFIGURATION Use. So it makes sense that the structure of the periodic table reflects periodic trends in the electron configuration of elements.
So Oxygens electron configuration would be O 1s22s22p4. There are main shells and subshells. The three colored lines represent a plot of the atomic radii of elements in the first three periods on the periodic table.
As you complete the activity keep the following in mind. Oct 01 2019 this downloadable color periodic table contains each elements atomic number atomic mass symbol name and electron configuration. Up to 24 cash back The atomic number of the elements on the periodic table are organized chronologically starting with Hydrogen with the the atomic number of 1 going from left to right.
Looking at the periodic table you can see that Oxygen has 8 electrons. As you go from left to right across the periodic table the atomic radii _. How many electron configurations does a chlorine atom have.
Describe and explain the pattern in electron configurations for the first 18 elements. The periodic table as an organisational tool to identify patterns and trends in and relationships between the structures including electronic configurations and atomic radii and properties including electronegativity first ionisation energy metallicnon-metallic character and reactivity of. Electron Configurations in the Periodic Table Four elements hydrogen carbon oxygen and nitrogen are the major components of most organic compounds.
Periodic patterns in electron configurations in each column write the element name and the electron dot diagram for the element that appears in that place on the periodic table see samples below group 1 group 2 group 13 group 14 group 15 group 16 group 17 group 18 period 2 lithium be b carbon n o f n e period 3 na mg ai si p s ci. The periodic table is arranged in order of increasing atomic numbers. Can hold no more then 2 electrons Lowest amount of energy and strongest attraction to the nucleus 2nd energy level Electrons will enter the second energy level only after the girt energy level is full Can hold no more then 8 electrons 3rd energy level Electrons will enter the 3rd energy level only after the 2nd one is full.
For example all the electron configuration of all elements in group 2 can be expressed in the form X ns² where X is the configuration of the noble gas from the preceding period and n is the principal quantum number. Later you will use these patterns to determine the order in which electrons fill the orbitals of an atom. Elements 11 to 18 follow the same pattern as elements 3 to 10 except electrons are placed in the third level instead of the second level.
The first main shell is 1 second. The arrangement or electron configuration forms an obvious pattern on the periodic table on the simply by looking at the periodic table. Metalloids have properties.
Electron Configuration Order of filling electrons in the orbitals and Shells When an atom or ion receives electrons into its orbitals the orbitals fill up in thefollowing order. The main shells are denoted by a number eg. Consequently our understanding of organic chemistry must have as a foundation an appreciation of the electronic structure and properties of these elements.
Atomic radii differ in an anticipated and logical way over the periodic table. Group 1 1 electron in outer level very reactive soft silver shiny low density. There are two electrons in the s subshell and six in the p subshell.
Mg 1s 22s2 2p6 3s 2. Based on the order of fill above these 8 electrons would fill in the following order 1s 2s and then 2p. The atomic number is the number of protons in the nucleus of an atom therefore it is the same to the charge number of the element.
Symbol e- Orbital Diagram and Longhand Electron Configuration 1. Use the patterns within the periodic table to draw orbital diagrams and write longhand electron configurations for the following atoms. Period row Group column Use the table on your book cover which shows only.
See Figure 1 Figure 1. In this activity you will identify these patterns. Lithium Sodium Potassium Rubidium Cesium Francium alkaline earth metals metallic elements in group 2 of the periodic table which are harder than the alkali metals and are also less reactive coinage metals copper silver gold Iron Triad iron cobalt nickel.
The electrons will always go to the lowest energy level possible. Write the electron configuration for the first 20 elements of the periodic table. With an atomic number of ten neon has two electrons in the main shell and eight electrons in the second shell.
Elements 3 to 10 fill 1s 2s and 2p totaling 8 electrons for the level. The main properties that can be compared is the melting. P 1s2 22s2 32p6 3s 3p 3.
Previous page with a little practice you will be able to find the electron configuration of any element Which groups are. Printable Periodic Tables Pdf Periodic Table Printable Source. Therefore its ground state electronic.

Electron Configurations In Atomic Energy Levels Video Lesson Transcript Study Com
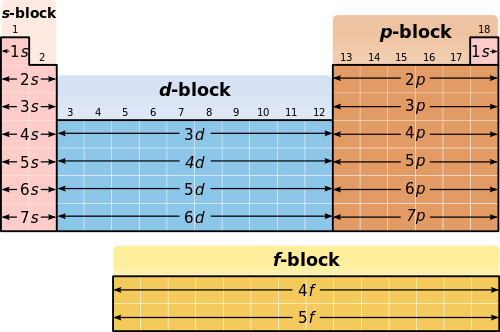
Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry

Writing Electron Configurations Using Only The Periodic Table Youtube

Electron Configuration And The Modern Periodic Table Examples Pedia

Electron Configurations The Cavalcade O Chemistry
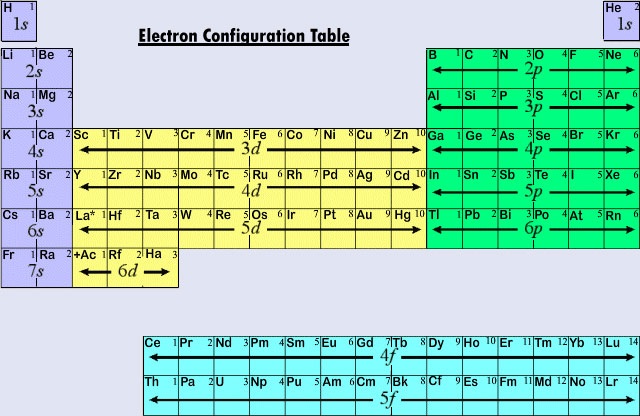
5 17 Electron Configurations And The Periodic Table Chemistry Libretexts

Dublin Schools Lesson Electron Configurations Using The Periodic Table

0 komentar
Posting Komentar